Message from the Core Directors
We hear you loud and clear!

Jane N. Bolin, RN, JD, PhD
Co-Director: Regulatory Safety & Compliance
As a researcher you want to conduct research not spend hundreds of hours traversing your way through the confusing labyrinths of research regulations, IRBs, IACUCs, often filling out numerous forms required by multiple institutions. We are a free resource with the goal of providing centralized research compliance support services for investigators.

Robert Nobles, DrPH, MPH, CIP
Co-Director: Regulatory Safety & Compliance
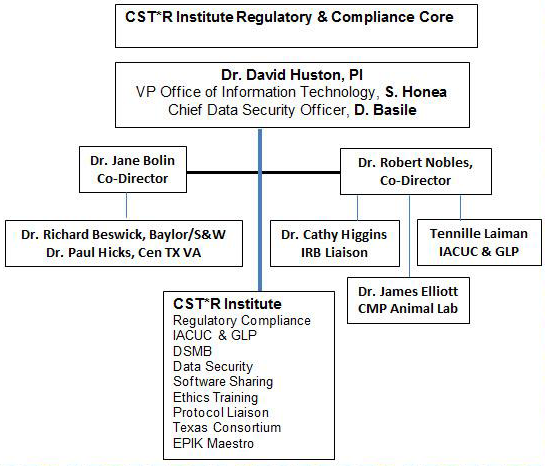
The mission of the CST*R Institute’s Research Compliance and Ethics Core is to provide an environment that facilitates interprofessional and trans-disciplinary research leading to the translation of medical discoveries, to testing and population-based application through the establishment of a unified compliance portal for research teams, and to facilitate the training coordination of research compliance. The Research Compliance & Ethics Core is responsible for assisting the TAMU Office of Research Compliance with all aspects of research compliance, including safety, training in human subjects’ research, care and protection of animals used in research, investigation of new drugs (IND) compliance, and ethics training/education.
CST*R’s Research Compliance Core supports the TAMU Office of Research Compliance and their staff of highly qualified professionals by providing affiliated researchers with a unified point of contact and portal for all CST*R investigators to access the necessary compliance requirements as well as a unified portal for training.
This program:
Provides leadership and guidance in compliance and regulatory affairs by facilitating the process of navigating multiple compliance review committees, thereby reducing regulatory barriers for translational researchers;
Enhances knowledge about human subject protection by providing regulatory education and training to investigators, students, and the public by collaborating with other CTSA programs, in particular the Texas CTSA consortium;
Increases research and regulatory support services for new, early-career investigators, particularly in protocol development, informed consent, development of data and safety monitoring plans, consultations with cGMP, IND/IDE submissions, and technology transfer.
Works with the State of Texas IRB and Texas CTSA Consortium leadership to develop state-wide uniform regulatory compliance agreements, MOU’s, and reciprocity documentation.
Enhances regional and national leadership through participation in the Texas CTSA Consortium, the NIH CTSA consortium and other national initiatives relevant to regulatory issues.
Provides Research Data Safety and Security for all members and partners of CST*R through the stringent application of federal and state laws, establishment of a Data Safety Monitoring Board (DSMB) and Chief Data Safety Officer (DSO) for data safety monitoring and protection of all data used in research.